The Berkeley Club Beverages, Inc. (a company based in Berkeley Springs, West Virginia) voluntarily recalled its bottled-water products labeled “Berkeley Springs Water Purified” and “Berkeley Springs Water Distilled” in September 2024 after test results indicated the presence of coliform bacteria.
According to Newsweek, the U.S. Food & Drug Administration (FDA) officially lifted the recall on November 13, 2024.
This is why you might have seen people searching for “Berkeley Club Beverages recall terminated” news.
The recall falls under the Class III category, indicating that the likelihood of serious health consequences from product exposure was minimal.
However, this incident remains noteworthy to consumers, the legal community, and those monitoring regulatory actions.
It raises questions of product safety, regulatory oversight, corporate compliance, and how consumers should respond when a recall is terminated.
In this article, we will delve into the following topics:
- Why did the recall happen?
- What was recalled?
- What do coliform bacteria mean in a regulatory and health context?
- How did the recall process function?
- Why was the Berkeley Club Beverages Recall terminated?
- What consumers should do now?
Stay tuned
Background: How The Recall Started?

- The recall ran approximately from September 12, 2024, to November 13, 2024.
- It was designated as a Class III recall by the FDA.
- The initial notice reported over 150,000 bottles; corrected later to about 1,300 bottles (discrepancy remains).
- The risk level was low, yet the presence of coliform bacteria signals potential hygiene/sanitation issues.
Detection Of Contamination:
Berkeley Club Beverages detected issues with its bottled water after internal or external testing flagged coliform bacteria in specific batches of its still-water products, according to Food & Wine.
Additionally, while the FDA notice did not publicly specify the exact testing protocol or the detailed pathway of contamination, the presence of coliforms in a bottled-water product triggered regulatory review.
According to media reports, the initial recall notice stated that the company “tested positive for coliforms” and voluntarily recalled the product.
The key point is that, even though the assessment of immediate health risk was low, coliform bacteria are indicators of a breakdown in sanitation or potential pathogen ingress, thus meriting a recall.
Voluntary vs. Involuntary Recall:
This recall was voluntary. Top Class Actions reported that Berkeley Club initiated the recall on September 12, 2024. In FDA terms, a “recall” can be:
(i) firm-initiated voluntarily.
(ii) at the FDA’s request.
(iii) FDA-mandated under its authority.
According to the FDA’s Regulatory Procedures Manual, a recall will be terminated when the agency determines “all reasonable efforts have been made to remove or correct the product … and proper disposition or correction has been made commensurate with the degree of hazard of the recalled product.”
Because it was voluntary and classified as Class III, the regulatory burden was lower than a forced recall.
Nonetheless, from a legal-compliance perspective, the company still had to coordinate with the FDA, provide recall status reports, and effect meaningful corrective action.
What Was Recalled? Products & Scope Of Berkeley Club Beverages Recall Terminated Issue
The products affected were the still-water lines of the company:
- Berkeley Springs Water Purified – 1-gallon & 5-gallon plastic containers.
- Berkeley Springs Water Distilled – the same sizing (1-gallon & 5-gallon).
Batch/Lot Codes:
The recall notice identified these specific codes: 090326, 090426, 090526, 090626. These lot codes were the key identifiers that consumers and retailers checked.
Distribution Area:
According to People.com, the bottles were distributed in three U.S. states: West Virginia, Maryland, and Virginia.
While early reports cited over 150,000 bottles (e.g., about 151,397) across those states, subsequent corrections indicate the actual number recalled may have been much lower (approx. 1,304 bottles) — though still the official recall remains with the higher figure in some reports.
From a legal-compliance standpoint, the scope of distribution matters for liability, jurisdiction, and consumer notification.
What Are Coliform Bacteria And Why Do They Matter?

“Coliform bacteria” are a broad group of bacteria that we can commonly find in soil, surface water, and vegetation. Additionally, we can also find it in the intestinal tracts of warm-blooded animals.
They serve as indicator organisms, meaning their presence can suggest the possible presence of fecal contamination or other pathogenic bacteria.
For instance, among them, you need to know about the following:
- Total coliforms: a large family, many not harmful per se.
- Fecal coliforms (a subset): more closely associated with fecal (animal or human) waste.
- Escherichia coli (E. coli): a specific species within the fecal coliform group; certain strains are pathogenic.
Health Risks:
Although many coliforms are benign, their detection in drinking or bottled water is a red flag. As reported:
“Even though the water bottles included in this recall may not directly cause harm … it’s probably better to be safe than sorry.” The presence of coliforms may indicate:
- potential contamination by more harmful bacteria (e.g., pathogenic E. coli)
- failure of treatment, sanitisation, or bottling hygiene processes
If pathogenic strains were present, symptoms might include diarrhea, vomiting, stomach cramps, fever, etc.
For vulnerable populations (children, the elderly, and immunocompromised), even low-level contamination can escalate.
Thus, while the risk in this particular recall was low, the detection triggered regulatory action as a precaution.
Regulatory Standards:
In the U.S., regulatory oversight for bottled water typically falls under the FDA’s food safety framework (and for municipal water, under the Environmental Protection Agency (EPA)).
For example, EPA’s guidance states that the total coliforms help determine the adequacy of water treatment and the integrity of the distribution system.
According to the regulatory manuals, the detection of coliforms triggers follow-up testing to determine whether E. coli is present.
For bottled water, while the exact standard may differ from public water systems, the presence of coliforms remains unacceptable and triggers removals or recalls.
FDA Classification: What Is A Class III Recall?
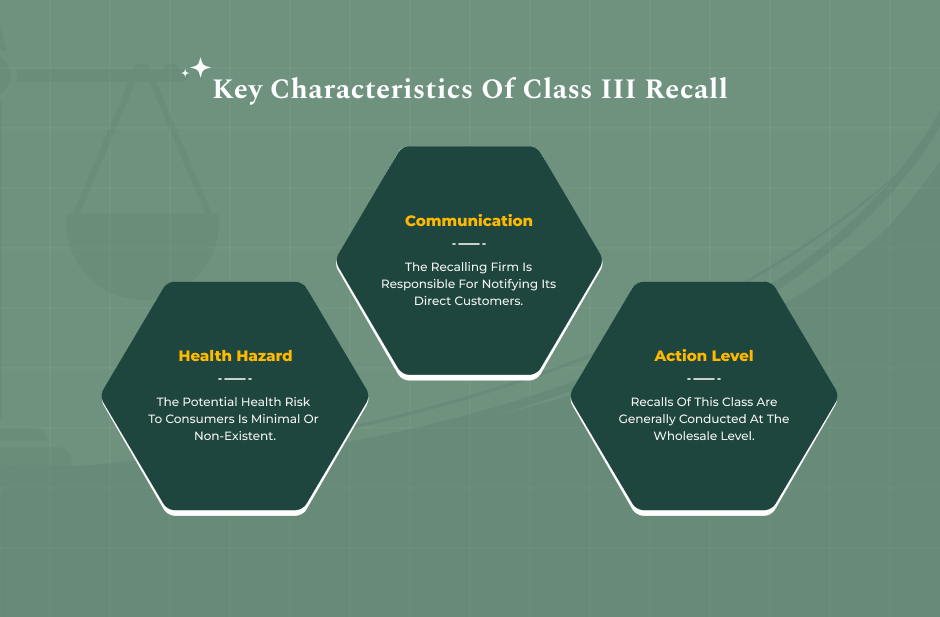
The FDA classifies recalls into three tiers:
1. Class I: “A situation in which there is a reasonable probability that the use of, or exposure to, a violative product will cause serious adverse health consequences or death.”
2. Class II: “A situation in which use of, or exposure to, a violative product may cause temporary or medically reversible adverse health consequences or where the probability of serious adverse health consequences is remote.”
3. Class III: “A situation in which use of, or exposure to, a violative product is not likely to cause adverse health consequences.”
Legal professionals must recognise that while a Class III is the lowest risk, it does not mean “no risk.” Rather, it means that the regulatory assessment deems serious harm unlikely.
The FDA designated the Berkeley Club Beverages recall as Class III on November 8, 2024.
The justification: although coliforms were detected, the exposure was assessed as unlikely to cause adverse health consequences in the general population.
Thus, the lowest risk classification applies. The classification helps determine the urgency of notification, communication strategy, and monitoring scope.
Furthermore, this classification has implications for liability, consumer expectations, and regulatory disclosure obligations. Many consumers may mistakenly believe “Class III” means “safe.”
However, the presence of coliforms still reflects a regulatory violation and hygiene failure.
Timeline: From Recall to Termination
Here’s a quick look at the timeline:
| September 12, 2024 | Start of Recall | Berkeley Club Beverages voluntarily initiated the recall of the four batches identified |
| November 8, 2024 | FDA Risk Classification | The FDA classified the recall as Class III |
| November 13, 2024 | Termination | The FDA terminated the recall of Berkeley Club Beverages’ water |
What Termination Means?
According to FDA guidelines, a recall is terminated when the agency determines that:
“all reasonable efforts have been made to remove or correct the product in accordance with the recall strategy, and when it is reasonable to assume that the product subject to the recall has been removed and proper disposition or correction has been made commensurate with the degree of hazard of the recalled product.”
In practice, termination means the recall is no longer active, the firm and regulators agree the corrective action is complete, and the product is no longer considered an active regulatory risk.
It does not mean the recalled product was perfect or that the incident posed zero risk.
For legal content writers, this is significant: termination signals regulatory closure but may not eliminate potential liability claims, especially if affected consumers later experience harm.
How Did The Berkeley Club Address The Issue?
Some of the things that Berkeley Club addressed about the termination of the recall are as follows:
1. Quarantine And Destruction:
Firstly, although detailed public disclosures from Berkeley Club are limited, media reports indicate the company quarantined, accounted for, and destroyed the affected bottles.
For example, a Newsweek report states: “the company… said most bottles were recovered, quarantined and destroyed.”
Additionally, from a compliance standpoint, disposal of the violative product is part of the “proper disposition” necessary for termination.
2. Quality Assurance Overhaul:
Secondly, while the company has not publicly detailed each corrective action, standard practices would include:
- Reviewing bottling line sanitation, water-treatment systems, and microbial testing protocols.
- Instituting enhanced sampling of finished product for coliform bacteria.
- Verifying lot coding, traceability, and distribution records.
According to Bible Aura, discussions of recall resolution and termination strategies highlighted these actions.
3. Regulatory Cooperation:
Thirdly, Berkeley Club, by initiating a voluntary recall, cooperating with the FDA classification, and achieving termination, appears to have successfully engaged in the regulatory process.
That said, actual regulatory inspections or public enforcement outcomes have not been widely publicised for this matter.
From a legal viewpoint, documentation of cooperation, recall strategy, corrective action logs, and audit checks is critical for potential liability defence or regulatory review.
Read Also: Progressive Class Action Lawsuit Settlement: How Can You Get A Part Of The Money?
Consumer Impact And Safety Guidance (Expert Tips):
Here are some of the things that consumers can do:
How To Identify Recalled Bottles?
- Check for the product names: “Berkeley Springs Water Purified” or “Berkeley Springs Water Distilled”.
- Size: 1-gallon and 5-gallon plastic containers.
- Batch/lot codes: 090326, 090426, 090526, 090626.
- Location: sold in West Virginia, Maryland, and Virginia (though consumers elsewhere may check as a precaution).
If your bottle matches all of the above, avoid consuming it.
What To Do If You Have Berkeley Club Beverages Recall Terminated Water?
- Stop using the product immediately.
- Dispose of it safely (pour it out, recycle/throw away the container) or return it to the supplier.
- Contact the manufacturer or retailer to ask about a refund or replacement (if the recall notice provides a contact).
- Monitor for symptoms of gastrointestinal illness; if you feel unwell, contact your physician and report to your local health department.
Who Might Be At Higher Risk?
While general consumers were assessed as low risk, certain populations face greater vulnerability:
- Infants and young children
- Elderly persons
- People with compromised immune systems or chronic gastrointestinal conditions
For these groups, even low-level indicators of contamination warrant extra caution.
Preventive Habits For The Future:
- Purchase bottled water from reputable brands with clear batch/lot codes and date stamps.
- Store bottled water in cool, clean conditions; avoid exposure to high heat or direct sunlight, which may compromise packaging integrity.
- Periodically check for recall notices (see below), especially if you store large quantities of water.
- Rotate stock—don’t let bottled water sit unused for very long.
- Be proactive: subscribe to recall alert services (e.g., FDA’s recall email alerts) and check batch codes of major purchases.
Read Also: Yellowstone Bison Herd Lawsuit: Why Did Montana Conservation Group Sue The National Park?
Broader Implications: What This Means For Industry And Regulation?
When it comes to future implications for the industry, here are a few things that you should know:
Regulatory Lessons:
Firstly, the Berkeley Club recall and termination highlight several regulatory takeaways:
- Even minor contamination indicators (coliforms) trigger formal recalls: Regulatory oversight remains active even when risk is low.
- Recall termination criteria and classification matter: Documenting effectiveness, traceability, and disposition is essential.
Additionally, even for beverages perceived as low-risk, it means that firms must maintain:
- Robust batch coding.
- Distribution records.
- Recall readiness.
Legal professionals should note how recall procedures (voluntary recall, classification, termination) align with the FDA’s Regulatory Procedures Manual.
Brand Reputation:
Secondly, for Berkeley Club Beverages, the recall represents a reputational event: although the health risk was low and no illnesses were reported publicly, consumer trust may be impacted.
Moreover, the quick and transparent handling, combined with termination, may mitigate long-term damage – but consumers and retailers may still view the brand with increased scrutiny.
Consumer Trust And Recall Transparency:
Finally, from a public policy perspective, the number of recalled notices (150,000 vs. 1,300 bottles) in this case underscores the importance of communication and data accuracy in building trust.
Additionally, misreporting at an early stage could lead to a loss of trust. Media reports say the FDA initially stated about 151,397 bottles, then corrected.
Going forward, companies should: publish recall notices promptly; include clear instructions for consumers; maintain visible contact channels; and track communication effectiveness.